All biological microscopists face tradeoffs in the attempt to increase contrast in their specimens. Carbon-based life forms are composed mainly of light elements, making specimens essentially transparent in the electron microscope. Heavy metal salts are traditionally added for positive staining of fixed and embedded specimens and negative staining of whole structures deposited on a support film.
Phase contrast light microscopy has long been an indispensible tool for live-cell imaging. As shown more than 60 years ago by Zernike, the insertion of a phase plate in the diffraction plane of the light microscope generates high contrast images. This effective technique has only recently been successfully applied to transmission electron microscopy.
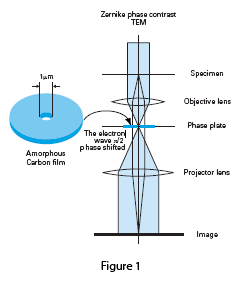
The column design for a Zernike phase plate TEM is shown in Figure 1. The phase plate consists of a continuous amorphous carbon film of appropriate thickness with a small central hole placed in the back focal plane of the objective lens. The zero-order beam passes through the central hole while scattered electrons are phase-shifted by -π/2, resulting in a three to five times increase in specimen contrast.
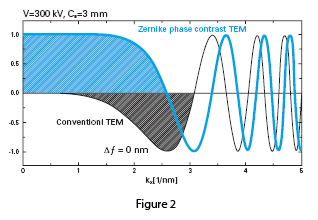
The effect of the phase plate can be demonstrated in Figure 2, a comparison of the contrast transfer functions (CTF) of conventional versus phase plate TEM. The CTF for a conventional TEM shown in black is a sine-type function, and low frequency components are greatly suppressed, generating low contrast in images of unstained samples. It is common practice to take data at optimum defocus or with a range of defocus settings to boost contrast. The CTF for the phase plate TEM shown in blue is a cosine-type function, with enhanced contrast of low frequency components. Phase plate images maintain high contrast near true focus, and no CTF correction is needed to interpret the images.
The lab of Professor Kuniaki Nagayama has championed the use of phase plates for TEM. He and his colleagues have illustrated the potential for phase contrast TEM imaging of low contrast, vitrified macromolecules. In the conventional TEM, images of frozen-hydrated or unstained biological specimens must be recorded with a high amount of defocus to produce sufficient phase contrast at low spatial frequencies to locate areas of interest. Since such imaging conditions sacrifice high resolution detail, alternative contrast enhancement methods such as the phase plate must be implemented.
The impact of phase contrast TEM on single particle analysis is multifold. Danev and Nagayama’s 3-D model of GroEL at 12Å resolution based on phase-contrast images required 30% fewer particles compared to a conventional image set, results especially critical for scientists examining heterogeneous samples, specimens available at low concentration or structures with low symmetry.
The optimal design for phase contrast cryo-electron tomography demands careful consideration of all optical elements to maximize image contrast, and features necessary in a dedicated column configuration have been incorporated in the JEM-2200FS. The JEM-2200FS 200kV TEM features a field-emission gun, a heated phase plate holder, an objective focal length of 5mm or larger to accommodate the phase plate holder, plus a room-temperature aperture to serve as a heat shield. The phase plate holder includes piezo drives for precision positioning and continuous heating of the phase plate up to 200ºC to minimize charging and contamination. The small size of the central hole of the phase plate requires accurate software control to center parallel illumination in all low dose modes and tilt angles. Zero-loss energy filtering provided by the in-column Omega filter roughly doubles the signal-to-noise ratio and is essential for removing inelastic scattering when imaging thick samples. The realization of this design in the JEM-2200FS should extend the practical examination of the vitrified proteome to include nanomachines as small as 100kDa.
For detailed information, please refer to the following articles and the brochure “Electron micrographs of phase contrast electron microscope”:
- F. Zernike (1942) Phase contrast, a new method for the microscopic observation of transparent objects. Physica 9: 686-698 and 974-986.
- R. Grimm et al. (1998) Improving image quality by zero-loss energy filtering: quantitative assessment by means of image cross-correlation. J. Microscopy 190: 339-349.
- K. Nagayama and R. Danev (2008) Phase contrast electron microscopy: development of thin-film phase plates and biological applications. Phil. Trans. Roy. Soc. B: 2153-2162.
- M. Yamaguchi et al. (2008) Zernike phase contrast electron microscopy of ice-embedded influenza A virus. J. Struct. Biol. 162: 271-276.
- R. Danev and K. Nagayama (2008) Single particle analysis based on Zernike phase contrast transmission electron microscopy. J. Struct. Biol. 161: 211-218.
- K. Nagayama (2008) Development of phase plates for electron microscopes and their biological application. Eur. Biophys. J. 37: 345-358.
- R. Danev et al. (2009) Practical factors affecting the performance of a thin-film phase plate for transmission electron microscopy. Ultramicroscopy 109 : 312-325.