What is cryo-electron microscopy used for?
Cryo-electron microscopy, or cryo-EM, is an imaging method that has revolutionized imaging of macromolecular complexes.1 Numerous atomic structures solved purely by cryo-EM and single particle analysis (SPA) have been deposited into data banks.
One of the older techniques in structural biology, protein x-ray crystallography, uses high energy photons that are diffracted from a high-quality crystal from different angles to build the 3D structure. However, not every protein can be crystallized and, although the drive to better beamlines allowed researchers to solve structures from ever smaller crystals, many samples are out of reach of this method
Cryo-EM by virtue of sub-millisecond vitrification does offer a way to obtain true or near-atomic resolution structures without requiring crystals. This approach has been highly successful on samples such as membrane proteins, reaction intermediaries, complexes that are fleeting, or those that exist in a mixture that can be heterogeneous in conformational and/or compositional in nature.
The field of structural biology was revolutionized with the advent of direct detectors cameras and highly stable and automated cryo-electron microscopes in cryo-EM. This resolution revolution propelled cryo-EM into becoming one of the most widely
used tools in structural biology. In 2017 the Nobel prize in Chemistry was rightly
awarded to Dr. Henderson, Dr. Dubochet and Dr. Frank for each of their contributions to the field of cryo-EM. Cryo-EM is currently applied to study variants of the SARS-CoV-2 virus, various membrane proteins and the immune system.
1 Understanding the structure of these species is key in understanding how some of these protein systems function, or malfunction in the case of disease, as well
as in identifying potential drug-binding targets for therapies.
What is cryo-electron microscopy
Cryo-EM involves vitrifying the sample and imaging the vitrified, or frozen-hydrated specimen in the electron microscope under cryogenic conditions in transmission mode. The electrons that are scattered by the sample form an image modulated by phase contrast, which is magnified onto a camera. Although electrons have a much shorter wavelength than visible light photons, the final resolution in cryo-EM is governed by many factors, some of which are the aberrations in the entire system, both optically and environmentally; others are the radiation sensitivity of the sample2.
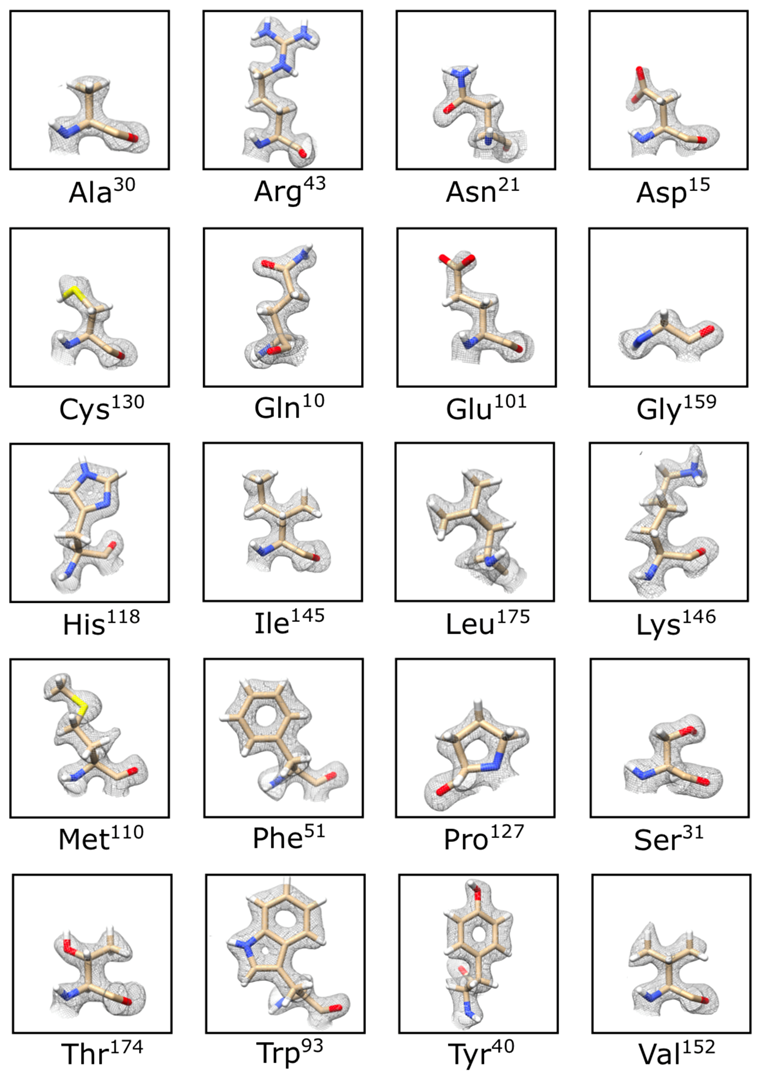
In SPA workflows, thousands of images of a macromolecular complex are recorded under low-dose conditions as movies on preferably a direct detector camera. Hundreds of thousands or even millions of particles are selected from motion-corrected movies and subjected to rounds of alignments, classification, and intermediate 3D model builds. After the final 3D reconstruction is scrutinized for correctness and resolution, the task is to try to understand the complex structure in terms of its functionality.
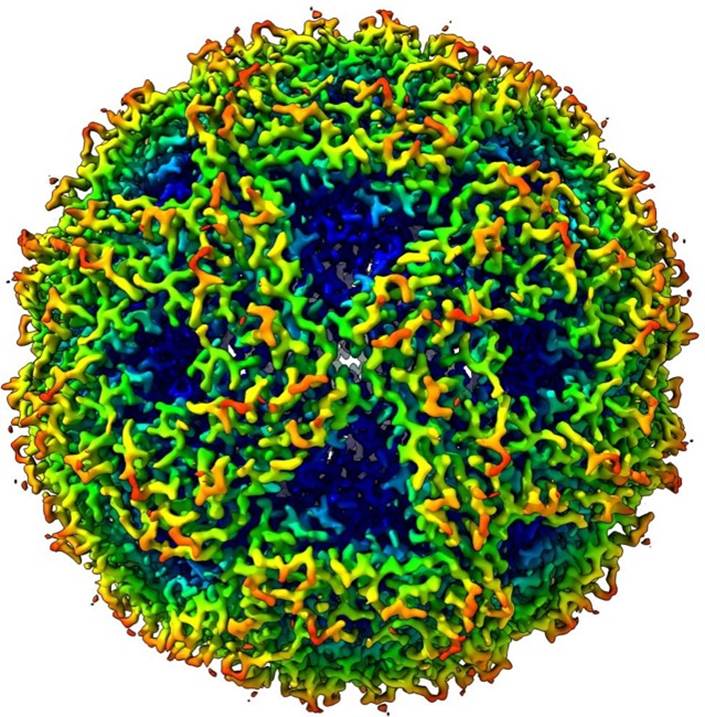
In tomography workflows, around 100 images are acquired under low-dose conditions from the same area of a vitrified sample at various tilts in the electron microscope. These images are recombined after corrections for tracking and focus changes into a 3D volume using a weighted-back projection scheme or a variant thereof. The resulting volume can be annotated and analyzed, but also subjected to sub-tomogram averaging techniques, whereby selected sub-volumes from the tomogram are processed using SPA techniques. Given that typically fewer sub-volumes are available the resulting 3D model of a complex is often obtained at a slightly lower resolution.
The biggest challenge in imaging vitrified specimens is radiation damage from the electron beam. This can show up in various forms, most easily recognizable as a loss of Bragg reflections of 2D crystalline samples, like catalase or purple membrane. However, other effects include charging as a result from secondary electron emission, bond breakage and even mass loss. Specimen charging is most often visible as beam-induced specimen motion and, in extreme cases, doming of the sample. These effects severely restrict any attempt to acquire a high resolution structure.4 Pioneering efforts at the MRC point to a new type of grid for SPA, hexafoils, to combat these effects with great efficacy5.
The success of cryo-EM applied to a macromolecular complex depends on many factors, some of which are biochemical in nature, others related to the vitrification process and yet others related to the automation on the microscope. Since many images are required to solve the structure of a complex, the best possible approach is to have as many images of the complex of interest as possible. Automation, gauged by the number of high resolution images per hour is important in any setting. A substantial fraction of the cycle time, i.e. the time to go from one area on the sample to the next, is taken up by either moving the stage and waiting for drift to settle, or simply the process of recording the movie. The current state-of-the-art puts this number around 20,000 movies/day. A clinical evaluation of all steps that factor into any throughput number is critical in the choice of equipment and imaging strategy.
JEOL cryo-electron microscopes
Performing successful cryo-EM means having the right microscope set up. JEOL offers the CRYO ARM 300 II, a dedicated instrument for highthroughput, automated cryo-EM using either SPA or tomography. The CRYO ARM 300 II will achieve this throughput via high levels of instrument stability and automated data collection through SerialEM or JADAS.3 With an ever expanding arsenal of experimental techniques and the availability of commercial cryo-electron microscopy instruments like the CRYO ARM 300 II, cryo-EM is projected to become the leading technique in Life Sciences in the coming years1.
References
- Callaway, E. (2020). Revolutionary cryo-EM is taking over structural biology. Nature, 578(7794), 201. https://doi.org/10.1038/d41586-020-00341-9
- Shen, P. S. (2018). The 2017 Nobel Prize in Chemistry: cryo-EM comes of age. Analytical and Bioanalytical Chemistry, 410(8), 2053–2057. https://doi.org/10.1007/s00216-018-0899-8
- Fislage, M., Shkumatov, A. V., Stroobants, A., & Efremov, R. G. (2020). Assessing the JEOL CRYO ARM 300 for high-throughput automated single-particle cryo-EM in a multiuser environment. IUCrJ, 7, 707–718. https://doi.org/10.1107/S2052252520006065
- Weissenberger, G., Henderikx, R. J. M., & Peters, P. J. (2021). Understanding the invisible hands of sample preparation for cryo-EM. Nature Methods, 18 (May), 463–471. https://doi.org/10.1038/s41592-021-01130-6
- Naydenova, K., Jia, P., & Russo, C.J. (2020) Electron cryomicroscopy with sub-Ångstrom specimen movement. Science October 9, 223-226. https://doi.org/10.1126/science.abb7927.