Why is the Sand Purple at Plum Island Beach?
The content of youTypical New England beach sand differs in color from light and dark grey to medium tan based on its common mineralogy, but at Plum Island Beach there are swatches of purple sand that appear haphazardly as one walks along the shore. The beach spans much of the length of Plum Island, an 8-mile long barrier island that takes a beating from the Atlantic Ocean in stormy weather. Tall dunes separate the beach from thickets, marshes, and a river that comprises the Parker River Wildlife Refuge established in 1942. At any given time, visitors can see seals on the beach, raptors, now including bald eagles, waterfowl, shore and song birds. In spring the beach is closed to allow Piping Plovers to nest. And in the mid-summer bravery is required to withstand the onslaught of the fierce biting green head flies.r post goes here. Drag any of the available blocks to create a stunning post and unleash your creativity:

When three coworkers from JEOL walked Plum Island Beach early in January they were hoping to spot a Snowy Owl perched high in the dunes where they have been known to appear in recent winters. Walking along the beach they noticed numerous bands of purple in the sand and wondered what had caused them: were they man made, pollution, rotting or decaying organic material or something in the sand's composition? They appeared randomly and yet frequently at the base of the dunes above the normal high tide line. One of the walkers gathered a handful of the purple sand and put it in his pocket to bring back to ask the company's geological experts for their opinion, and to have it analyzed using one of the JEOL scanning electron microscopes (SEM) and energy dispersive X-Ray spectrometers (EDS) with a little bit of optical microscopy prior to introduction into the SEM/EDS. JEOL USA, Inc. located in Peabody, MA, supplies much of the research world with SEMs that make it possible to see things at extremely high magnifications and also analyze them for their chemical composition.
At first look under the optical microscope, the granules of sand appeared like scattered jewels of many colors; predominantly glassy pink angular grains, with smaller quantities of milky white rounded grains, clear angular grains, black grains (some magnetic and some not), and even the occasional green.
What could be the cause of the purple color? The answer was one that came as no surprise to the scientist, but was exciting for the beach walkers because they had an exact answer to a question that no doubt is one that many people have when they visit Plum Island - which was actually named for its beach plum bushes, not the plum-colored sand.
When large amounts of fine grained pink is intermixed with a smaller number of darker grains and dampened by rain or sea water the human eye will “see” the sand as a much darker pink to almost purple. The two most common pink minerals are rose quartz (while quartz is one of the two most common minerals on earth, the pink rose quartz variety is not so common ,especially in the New England geology, and is found only in a few isolated pegmatite deposits in NH & southern Maine which are where most gemstones originate) and the solid solution series of almandine and pyrope garnet which is also a very common mineral (and is quite common in the Seacoast area from the abundance of metamorphic rocks called mica schist and from contact metamorphism. This is also why many commercial sandpaper products have a pink color as the angular hard gains of almandine / pyrope garnet are the perfect abrasive. The most likely candidates for the white and clear are any of the feldspars and or quartz. The green is most likely epidote. Just based on the optical examination these are no more than educated logical guesses (but still guesses).
Vern Robertson, JEOL’s SEM Technical Sales Manager, originally examined the grains under a low power optical stereo microscope with the above conclusions. In addition to providing technical and scientific support to JEOL SEM customers for a multitude of applications, Vern holds a degree in Geology. After a cursory look optically, it was time to get down to some spectroscopic analysis to determine the actual mineral species present in the sand.
Individual grains of various colors were selected and mounted for examination with the JSM-6010LA+ InTouchScope SEM and for analysis using EDS. The SEM allows much higher magnification imaging with greater depth of field than a traditional OM and the low vacuum capability allows examination of the sample without the traditional conductive coating that needs to be applied for SEM imaging. However, it generates images in only black & white (electrons have no color!). One specialized detector in the SEM, the Backscatter Electron Detector, yields images with the gray level intensity directly proportional to the average atomic number (or density). This means that minerals containing only lighter elements like O, Si are darker in appearance to minerals that contain heavier elements like Fe or any of the metallic or rare earth elements.
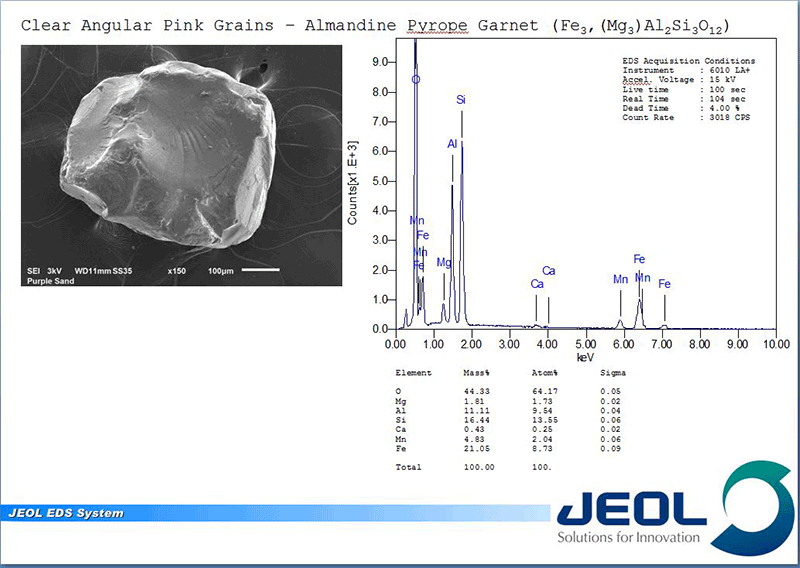
Once located, each grain can be analyzed with the EDS. When an electron beam hits a sample it creates not only an image from the emitted electrons but creates X-rays, which when collected in a spectrum, indicate what elements are present and at what concentrations. This allows not only the elemental composition of the individual grains to be determined but the concentrations can be compared to known stoichiometry of the suspected mineral grains. The combination of color and magnetic properties from OM examination and the chemical makeup of the individual grains yield the answer.
The purple color (or more appropriately, pink color) comes from the abundance of almandine-pyrope garnet with a nominal solid solution composition of Fe3+2Al2Si3O12 to Mg3+2Al2Si3O12. As expected, the white grains are a mix of feldspars but mostly K-feldspar (potassium alumino-silicates) and quartz SiO2. The black nonmagnetic grains were a mix of a pyroxene called augite which showed its characteristic strong cleavage, (Ca,Na)(Mg,Fe,Al)(Si,Al)2O6 , and a mix of ilmenite FeTiO3 and hematite Fe2O3 which are the magnetic components. The green was confirmed to be epidote Ca2(Al,Fe)3(SiO4) 3(OH). With the exception of the high concentration of garnets the rest are common minerals one would expect to find in sands.
While analyzing loose, irregular, as-received grains is not optimal for quantitative elemental analysis (high precision and accuracy quantitative analysis requires the samples be: clean, flat, polished, homogeneous at the scale being analyzed, and compared element by element to known similar matrix standards) the resulting standardless EDS analysis produced results that matched the known stoichiometry for these minerals nearly exactly.
The other item of note is that the sand is very angular and not rounded like one would expect in surf-tossed, coastal, oceanic beaches where the constant grinding of the grains by the tides would remove all sharp edges over time producing a “frosted” appearance like sea glass. Plum Island is a barrier island. This purple sand is high above the high tide high water mark and is a remnant of the initial deposition when the last glacial ice age began to recede and melt and dropped the sediments it had accumulated and pushed forward during its advance. These sediments likely come from tens to hundreds of kilometers away from their current resting place.
The Seacoast area is full of evidence of glaciation if you know what to look for like: HUGE boulders in a seemingly odd place pushed & dropped there by the massive ice sheets, shiny, striated, polished rock faces from the immense rubbing pressures as the glaciers overtook the land, barrier islands like Plum Island and even Boars Head at Hampton Beach, NH which is a terminal moraine or the farthest point the glacier reached on its southward march before retreating and leaving a pile of debris much like the snow plows of this winter would have done.
The Scanning Electron Microscope with an Energy Dispersive X-ray spectrometer (SEM/EDS) is a powerful tool not only in academia and industry but also in answering the vexing questions of: who, what, where, when & how of items encountered in everyday life.